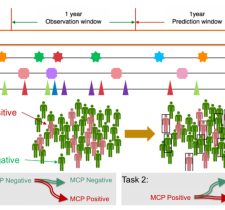
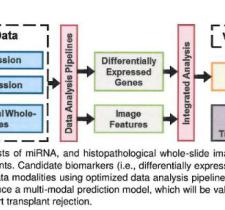
AI and machine learning have entered the mainstream lexicon and are of increasing utility in the healthcare continuum. But not all data need AI or ML to identify a problem and an opportunity for positive impact. Georgia Tech is a leader in the use of “big data” to solve big problems, including information from new bedside sensing technologies and Medicaid data from all 50 states. Medical sensors and devices can provide a flood of direct information about individual patients. Enormous amounts of data are also available from medical records of millions of patients in our state, region, nation, and world. Learning how to extract useful insights from such sources without compromising patient privacy is one of the most important areas of pediatric biomedical research today.
Equity is the absence of systematic disparities in healthcare delivery (e.g., access, quality, cost and outcomes) between groups of people. …