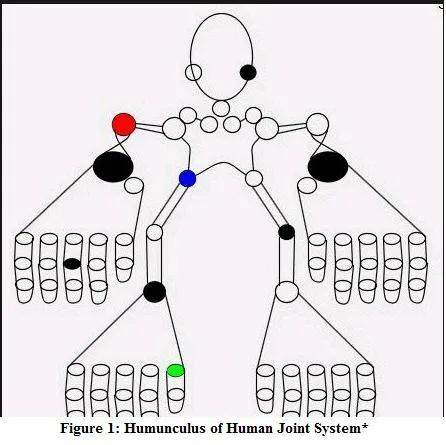
We designed and developed a homunculus based mHealth application for iOS to manage chronic arthritis in children from 7 to 18 years. To facilitate…
PI
Jiten Chhabra & Prabhu Shankar
Publications
Prabhu Shankar, MD, MS, Jiten Chhabra, MD, MS, Rob Solomon, MS, Sampath Prahalad, MD, MSc, Pediatric Healthcare Innovation: Advancing Technologies to Improve Child Health Conference, Georgia Tech Hotel and Conference Center April 22, 2014.
Research Area

